FDA 21 CFR Part 11 Audit and Assessments; we are ready when you are! Call +1 (888) 896-7580 today.
Table of Contents
Toggle
Title 21 CFR Part 11 is the portion of the Code of Federal Regulations that provides standards determined by the Food and Drug Administration (FDA) on electronic records and electronic signatures. With electronic records widely used in the Life Sciences industry, most companies will find FDA 21 CFR Part 11 applicable.
Regulated companies with documents or records in electronic format must comply with FDA 21 CFR part 11. Part 11 pertains to pharmaceutical companies, manufacturers of medical devices, biotechnology companies, CROs, biologics developers, and other companies regulated by the FDA.
Part 11 helps companies safely maintain data securely so that it is not lost or corrupted, ensures companies are implementing systems and software correctly, makes sure there are data-trace changes, and prevents falsified records.
The professionals at Lazarus Alliance are completely committed to you and your business’s FDA 21 CFR Part 11 audit success. Regardless of whether you represent the private sector or the public sector, we stand ready to partner with your organizations.
Why Work With Lazarus Alliance For An FDA 21 CFR Part 11 Audit?
The Lazarus Alliance FDA 21 CFR Part 11 compliance assessment, audit, and readiness gap assessment services help organizations ensure that electronic records and electronic signatures are trustworthy, reliable, generally equivalent substitutes for paper records and traditional handwritten signatures, and that those functions are in conformance with the requirements of 21 CFR Part 11 compliance.
Lazarus Alliance's cyber security audit professionals evaluate an organization's use and documentation of electronic records and electronic signatures as governed by applicable regulatory requirements. The result of a 21 CFR Part 11 compliance assessment determines the effectiveness of an organization's process within a highly regulated environment and suggests appropriate remedial actions as necessary.
Our Cybervisors will proactively and collaboratively identify risk exposures that threaten your organization, call +1 (888) 896-7580 to get started. — Michael Peters, CEO & Founder
Our audit delivery tool, Continuum GRC, streamlines the audit process, helps reduce the complexity of compliance efforts, and gives our clients the ability to combine multiple audit frameworks into one audit.
Lazarus Alliance’s primary purpose is to help organizations attain, maintain, and demonstrate compliance and information security excellence in any jurisdiction. Lazarus Alliance specializes in IT security, risk, privacy, governance, cyberspace law and FDA 21 CFR Part 11 audit compliance leadership solutions and is fully dedicated to global success in these disciplines.
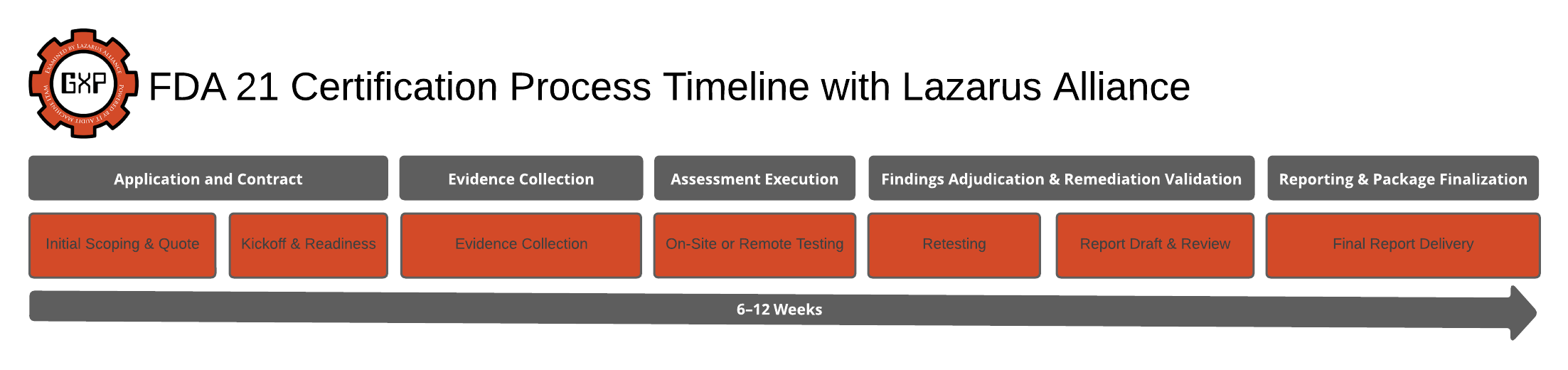
Basic Timeline for FDA 21 CFR Part 11 Audit Services from Lazarus Alliance
Lazarus Alliance's FDA 21 CFR Part 11 audits focus on ensuring electronic records and signatures meet regulatory standards for trustworthiness and reliability. While specific timelines vary based on organization size, system complexity, and scope (e.g., number of systems audited), a typical engagement follows a structured, risk-based process using their Continuum GRC platform for efficiency. Based on standard industry practices for Part 11 audits and Lazarus Alliance's described approach, here's a basic timeline outline. This assumes a mid-sized life sciences client with 5–10 systems in scope; actual durations may range from 4–12 weeks total.
| Phase | Description | Estimated Duration | Key Deliverables |
|---|---|---|---|
| 1. Initial Scoping & Planning | Kickoff call to define audit scope, system inventory, and risk assessment. Review predicate rules (e.g., GMP, GCP) and gather preliminary docs like SOPs and user access lists. | 1–2 weeks | Scoped audit plan, system inventory, and initial risk assessment. Schedule interviews and document requests. |
| 2. Document Review & Gap Assessment | Analyze validation packages, audit trail configs, electronic signature policies, training records, and recent internal audits. Identify gaps in controls like access, change management, and data archival. | 2–3 weeks | Gap analysis report highlighting common findings (e.g., inadequate audit trails or validation docs). Recommendations for quick wins. |
| 3. On-Site/Remote Audit Execution | Conduct user interviews, system demos, and evidence reviews (e.g., screenshots of audit trails, procedural docs). Evaluate security, procedural effectiveness, and regulatory significance using Continuum GRC for streamlined data collection. | 1–2 weeks | Interview notes, evidence logs, preliminary observations on compliance effectiveness. |
| 4. Analysis & Reporting | Synthesize findings, assess remedial actions needed (e.g., remediation roadmaps for hybrid systems or cloud oversight). Prioritize based on risk to product quality/safety. | 1 week | Final audit report with findings, remediation roadmap, and mock inspection insights. Attestation support if applicable. |
| 5. Follow-Up & Closure | Review remediation plan implementation, provide training/guidance, and confirm closure of high-risk gaps. Optional post-audit support for FDA inspections. | 1–2 weeks (or ongoing) | Closure verification, updated policies, and compliance certification prep. Schedule for periodic reviews (e.g., quarterly). |
Total Estimated Time: 6–10 weeks for a full audit cycle. Expedited options (e.g., mock audits) can take 4 weeks. Lazarus Alliance emphasizes proactive, continuum-based audits to minimize disruptions—contact them for a complimentary scoping call to tailor this to your needs. This timeline aligns with the FDA's risk-based expectations and helps avoid common pitfalls like neglected audit trail reviews.
Frequently Asked Questions
What is the primary focus of an FDA 21 CFR Part 11 audit?
21 CFR Part 11 is the FDA regulation that defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. A Part 11 audit assesses whether your systems that create, modify, maintain, archive, retrieve, or transmit electronic records (e.g., LIMS, EDC, eTMF, QMS, ERP) comply with the requirements for validation, audit trails, electronic signatures, record retention, and system security.
Does Part 11 apply only to systems used in clinical trials?
No. Part 11 applies to any electronic records that are created, modified, maintained, archived, retrieved, or transmitted under any FDA predicate rule (GMP, GLP, GCP, GTP, etc.). This includes manufacturing batch records, laboratory data, adverse event reporting, labeling systems, complaint files, and submission data.
We are moving to the cloud (SaaS). Are we still responsible for Part 11 compliance?
Yes—100%. Responsibility for Part 11 compliance cannot be delegated to your cloud or SaaS vendor. Even if your provider is validated and issues an IQ/OQ or certification, you remain ultimately accountable. You must perform supplier audits, review their SOC 2 + Part 11 bridge letters, and maintain risk-based oversight.
What are the most common Part 11 audit findings Lazarus Alliance sees in 2024–2025?
- Missing or inadequate audit trail review procedures
- Audit trails not enabled or not capturing meaningful data (who, what, when, why)
- Inadequate validation documentation for configured/commercial-off-the-shelf (COTS) systems
- Lack of electronic signature manifestations and linkage to records
- Open systems without proper encryption and access controls
- Poor change control and deviation management for validated systems
How often should audit trails be reviewed under Part 11?
The regulation does not prescribe a specific frequency, but the review must be risk-based and periodic. FDA expects reviews before final approval or release of records (e.g., batch release, clinical study data lock) and at least during routine quality/system reviews. Many companies adopt monthly or quarterly reviews plus event-triggered reviews.
Can we still use hybrid systems (paper + electronic) after the 2023 FDA guidance changes?
The FDA’s 2023 draft guidance on Part 11 and Data Integrity strongly discourages new hybrid systems and encourages full electronic workflows. Existing hybrid systems are grandfathered but must have robust controls to ensure the paper record is an exact copy of the electronic source. Most auditors now expect a roadmap to eliminate hybrid approaches.
Do spreadsheets used for GxP calculations need to comply with Part 11?
Yes—if the spreadsheet is used to make GxP decisions (e.g., stability data analysis, dose calculations, batch release testing). They are considered electronic records and typically fall under Part 11 “closed system” requirements. Controls include access restriction, version control, audit trails (via protected sheets or add-ins), and validation/testing of formulas.
What should we prepare before a Lazarus Alliance Part 11 audit or gap assessment?
- System inventory with Part 11 scope determination
- Validation documentation packages (including risk assessments)
- Audit trail configuration screenshots and sample reviews
- Electronic signature policies and training records
- User access lists and periodic review of evidence
- SOPs for system administration, change control, and data archival/retrieval
- Recent internal audit or self-inspection reports
Having these items organized in advance significantly reduces audit time and findings.
Lazarus Alliance specializes in proactive Part 11 continuum audits, remediation roadmaps, and FDA mock inspections for life sciences and medical device companies. Contact us for a complimentary Part 11 scoping call.
Credentials You Can Count On
American Association for Laboratory Accreditation (A2LA) ISO/IEC 17020 accredited certification number 3822.01.

Talk with one of our experts
Our Lazarus Alliance Cybervisor™ teams have experience performing thousands of assessments for organisations providing services to clients around the world.
We're here to answer any questions you may have.

Key Benefits of FDA 21 CFR Part 11 Compliance
(Real-world advantages for life sciences, pharmaceutical, biotech, and medical device organizations)
| # | Benefit | Business & Regulatory Impact |
|---|---|---|
| 1 | Avoid FDA 483s, Warning Letters, and Import Alerts | Non-compliance with Part 11 is one of the top 5 most frequently cited issues during FDA inspections. Full compliance dramatically reduces the risk of regulatory action, application refusals (RTF), or clinical holds. |
| 2 | Faster FDA Review and Product Approvals | Submissions containing trustworthy electronic records and signatures (eCTD, EDC, eTMF) move through review queues faster. Incomplete or questionable data triggers lengthy FDA information requests. |
| 3 | Significant Cost and Time Savings | Eliminates hybrid paper/electronic workflows, manual transcriptions, wet-ink signatures, physical storage, and courier costs. Fully electronic processes can reduce batch release cycles by 30–70%. |
| 4 | Stronger Data Integrity and Reduced Fraud Risk | Enforced audit trails, secure electronic signatures, and access controls make intentional or accidental data manipulation detectable and preventable—directly supporting ALCOA+ principles. |
| 5 | Improved Operational Efficiency and Quality | Validated systems with automated workflows, real-time audit trails, and electronic approvals reduce errors, speed up deviation investigations, and enable predictive quality analytics. |
| 6 | Global Regulatory Acceptance | Part 11–compliant systems are generally accepted by EMA, PMDA (Japan), Health Canada, ANVISA, and WHO because they exceed or align with ICH, EU Annex 11, and PIC/S requirements. |
| 7 | Easier Audits and Inspections (FDA, Notified Bodies, Partners) | Well-documented validation, routine audit-trail reviews, and clear system governance turn inspections from painful events into routine confirmations of compliance. |
| 8 | Stronger Market Confidence and Investor Trust | Demonstrating robust Part 11 compliance signals mature quality systems—critical for partnerships, due diligence in M&A, and attracting venture or public investment. |
| 9 | Future-Proofing for Emerging Technologies | Systems built on Part 11 principles (validation, audit trails, security) are ready for AI/ML in manufacturing, blockchain for supply chain, real-world evidence platforms, and decentralized clinical trials. |
| 10 | Reduced Legal and Financial Liability | In product liability or recall situations, provable data integrity and secure electronic records provide powerful legal defensibility (“our records are trustworthy by design”). |
Bottom Line
FDA 21 CFR Part 11 compliance is not just a regulatory checkbox—it is a strategic business advantage that protects revenue, accelerates time-to-market, cuts operating costs, and builds unbreakable trust with regulators, partners, and patients.
Lazarus Alliance helps organizations turn Part 11 compliance into these tangible benefits through proactive audits, remediation roadmaps, and Continuum GRC automation. Contact us for a free Part 11 health check.
